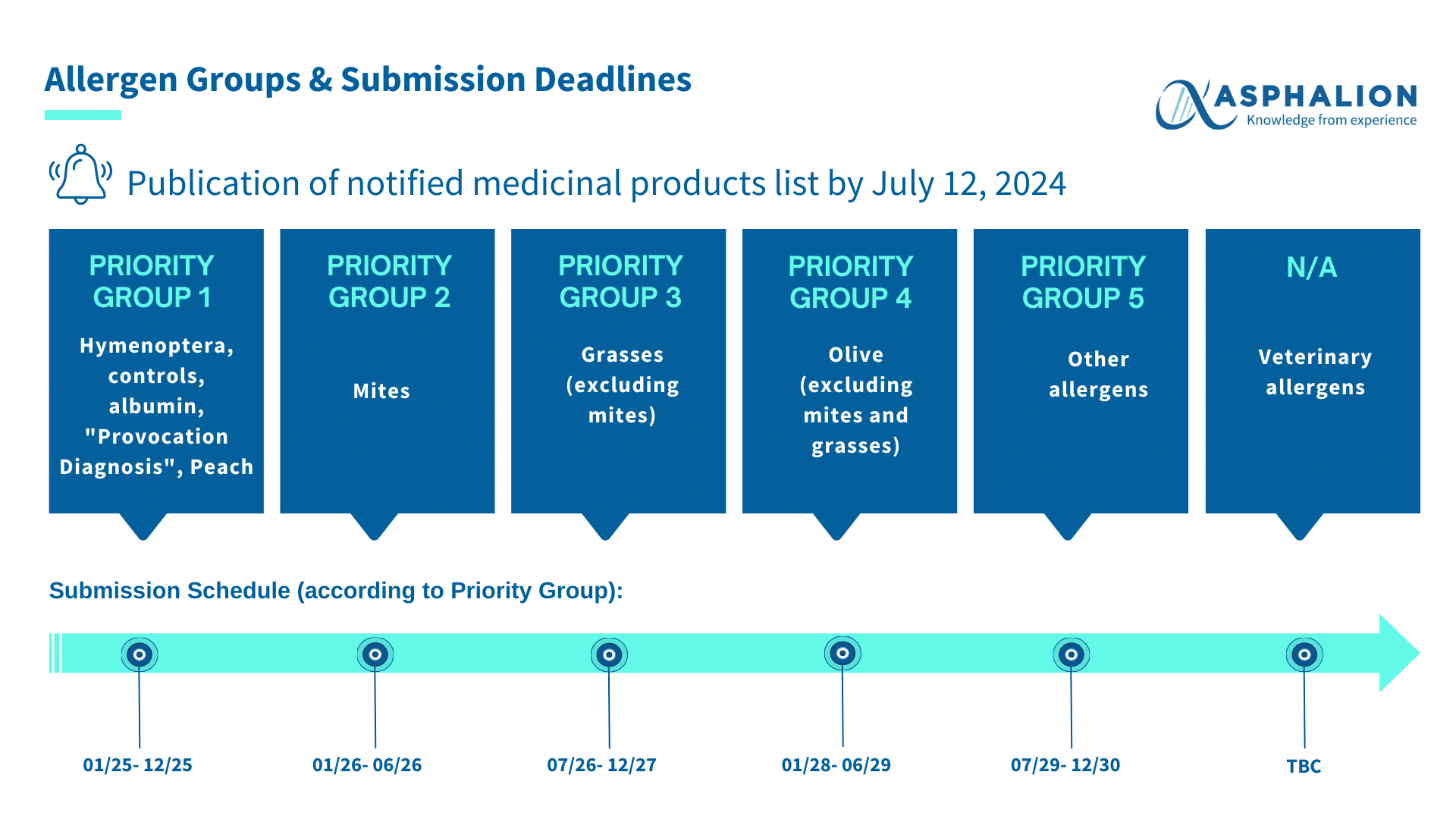
The Spanish Agency of Medicines and Medical Devices (Agencia Española de Medicamentos y Productos Sanitarios) published on July 12, 2024, the list of notified medicinal products, which includes a total of 17,465 notifications for allergen-based products.
Have a look at the detailed list of medicinal products for human use and the associated laboratories.
Please note that products not included on this list are not eligible for the specific regulatory process established by the AEMPS to obtain marketing authorisation (MA) at reduced costs and according to the established calendar.
At ASPHALION, we offer comprehensive and personalized regulatory support tailored to meet AEMPS requirements, including:
- Preparation of allergen-based dossiers
- Scientific and CMC writing
- Scientific advice
- Regulatory strategies
- TAC laboratory management
For more information, contact us at: [email protected]