Asphalion is excited to announce an upcoming transition that will significantly impact the submission process for paediatric procedures within the European Medicines Agency (EMA). Starting from June 4, 2024, the EMA will transition from the current EMA Gateway to the IRIS portal for submissions related to paediatric procedures.
This change will encompass the following submissions:
- Initial Paediatric Investigation Plan (PIP)
- Modification of an agreed PIP
- Product-specific waiver
- Compliance check
- Annual report on paediatric deferred measures
- Confirmation of applicability of a class waiver
- Discontinuation of Paediatric Development
The move to the IRIS portal promises a myriad of benefits, including faster interactions with the EMA, enhanced data quality through integration with other EMA systems, and the ability to monitor the status of applications in real-time from any device. These improvements are designed to increase transparency and efficiency, streamlining the paediatric application process for all stakeholders.
It is relevant to note that in addition to the current template-supported document package provided to EMA, it will be necessary in IRIS to include clinical and quality data associated with the paediatric procedure and formulation development in line with what is already requested by the existing templates.
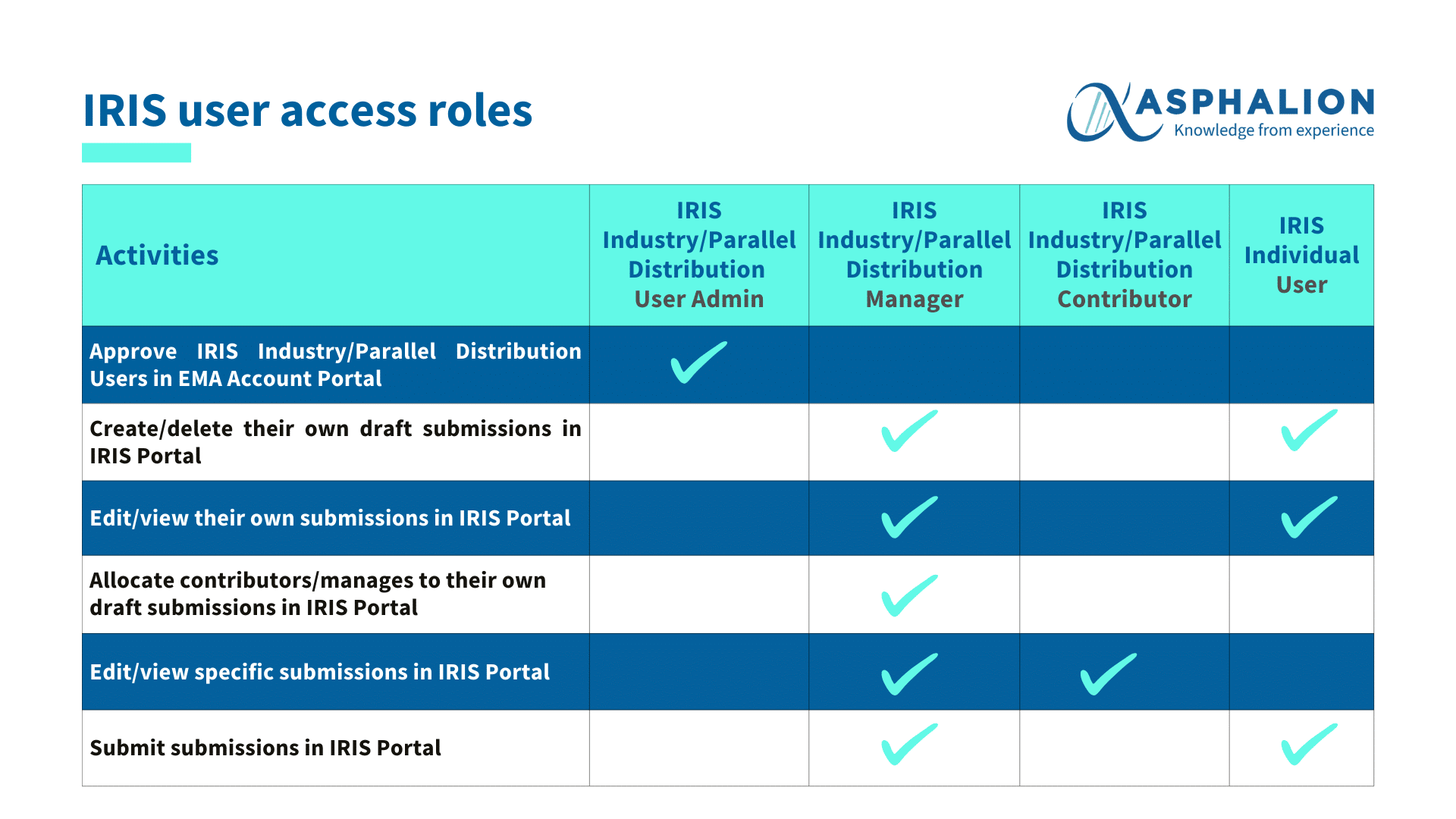
To facilitate a smooth transition, it is crucial for companies to prepare by ensuring they have an active EMA account. The IRIS platform offers various roles tailored to different functions within the application process, from Industry User Admin to Industry Coordinator, each with specific capabilities to facilitate submission management.
Paediatric procedures that started in advance of the 4th June go-live date will need to be concluded through the IRIS portal.
As a reminder, your company must be registered in SPOR to engage with the IRIS portal.
For any questions or assistance with IRIS or other regulatory processes, please do not hesitate to contact us at: [email protected]
Our team is here to support your company through these changes, ensuring compliance and continued success in the pharmaceutical landscape.