Clinical Data Disclosure Services
Empowering pharmaceutical breakthroughs with Clinical Data Disclosure Services for regulatory compliance and innovation
The pharmaceutical industry is moving towards greater transparency, driven by requirements of regulatory authorities like the EMA and Health Canada.
Regulators aim to promote innovation and new medicine development, while boosting public trust and confidence, by granting access to clinical information.
Asphalion is embracing the evolving transparency landscape. We lead the data disclosure revolution and offer customized services to navigate transparency policies.
Tailored Clinical Data Disclosure Services
Our Data Disclosure Services are designed to streamline the intricacies of transparency requirements, providing to our clients the support they need to meet their transparency obligations with precision and agility to achieve their goals efficiently.
Asphalion’s specialists can provide support across different policies:
EMA policy on publication of clinical data for medicinal products for human use -POLICY/0070
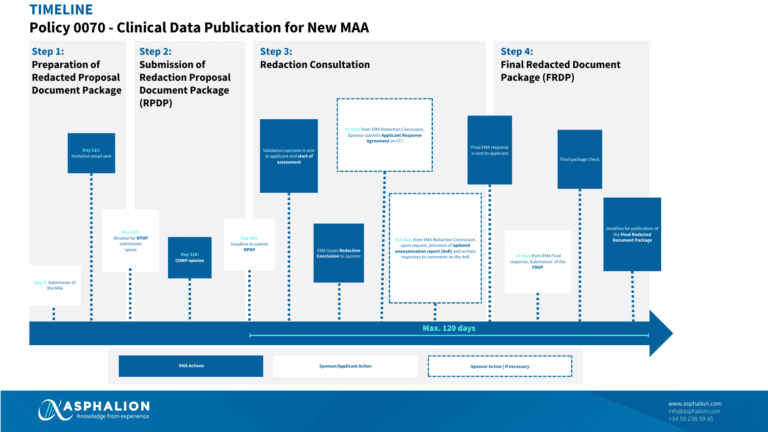
The EMA’s Policy 0070 on Clinical Data Publication (CDP) consists in the publication of clinical data submitted by pharmaceutical companies to support their request for marketing authorisation (MA), and which are assessed by the Committee for Human Medicinal Products (CHMP) via centralised procedure. The publication is due following the adoption of the scientific committee Opinion/conclusion or the receipt of the applicant withdrawal letter.
The Policy became effective on 1 January 2015 for any new centralised marketing authorisation procedure, and on 1 July 2015 for extension of indication and line-extension applications. On 5 December 2018, the Agency suspended the publication of clinical data. During the COVID-19 pandemic, the EMA reactivated the publication of clinical data for COVID-19 treatments and vaccines as an exceptional measure to maximize the transparency on these medicines, to address the high interest for information and to support global research.
The EMA is gradually resuming clinical data publication activities (beyond COVID-19 medicines): for the time being, the “step 1” of the re-launch has been implemented considering MA applications for new active substances (NAS), that received a CHMP opinion or were withdrawn from September 2023 onwards. “Step 2” is expected to be activated for other applications apart from new active substances and Covid-19 treatments during 2024.
Clinical data in scope of the policy are defined as clinical reports and individual patient data (IPD). Clinical reports correspond to modules 2.5, 2.7 and 5.3 of the common technical document (CTD).
Clinical reports must be anonymised to prevent patients and professionals who participated in clinical trials from being identified. The approach followed must be described in the Anonymisation report, compiling the risk assessment technique, the anonymisation methods used and their impact on data utility.
Companies must also justify the redaction of any commercially confidential information (CCI) and agree with the EMA on the pieces of information claimed as CCI during the procedure, including qualitative and quantitative risk assessment where applicable.
The timeline below summarises the process for an initial MAA:
Health Canada's Public Release of Clinical Information (PRCI)
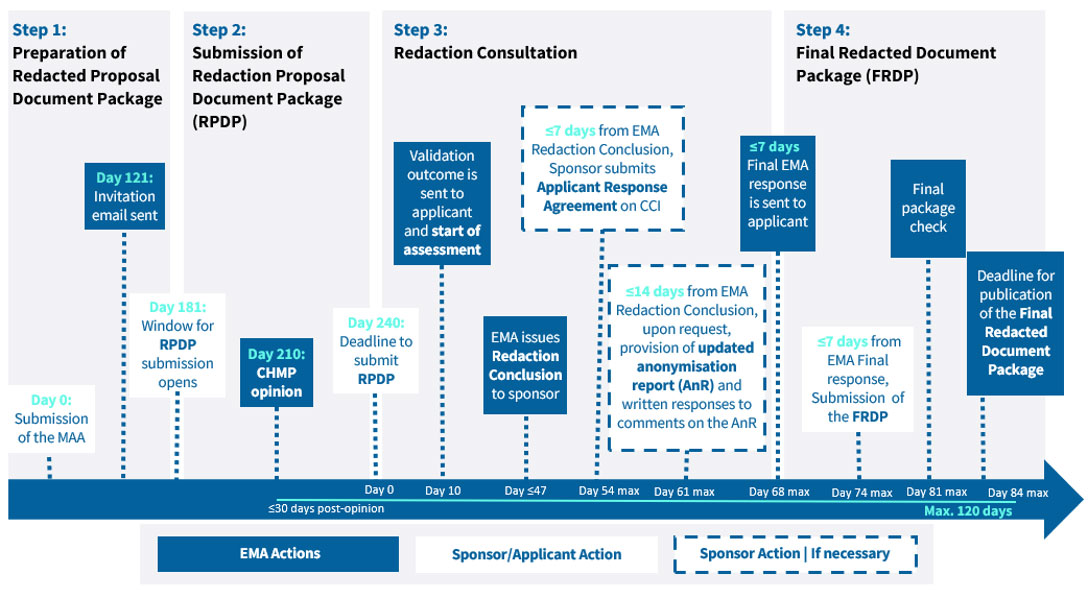
Health Canada’s PRCI initiative came into force on 2019 with the objective to make anonymized clinical information in drug submissions and medical device applications publicly available for non-commercial purposes following the completion of Health Canada’s regulatory review process, while adhering to Canada’s Privacy Act.
Proactive publication was phased in by submission type, until being applicable to all New Drug Submissions (NDS), Supplemental NDS (SNDS), Abbreviated NDS (ANDS), Supplemental Abbreviated NDS (SANDS) and Class III & IV devices. Information in drug submissions not subject to proactive release is available on request through Health Canada’s clinical information portal.
Health Canada aims to publish the final redacted and anonymized clinical information package within 120 calendar days from initiation of the process.
The documentation in scope of publication is the clinical information contained in eCTD modules 2.5 (Clinical Overviews), 2.7 (Clinical Summaries) and 5.3 (Clinical Study Reports).
The manufacturer is required to anonymize the clinical information using a risk-based anonymization process that must be detailed in a separate Anonymization report to ensure the risk of disclosing personal information can be reliably reduced. Confidential Business Information identified by the manufacturer can be proposed for redaction provided they are accompanied by specific and detailed justification.
EU Clinical Trials Regulation, Regulation (EU) No 536/2014
The Clinical Trials Regulation (CTR) entered into application on 31 January 2022 to replace the Clinical Trials Directive 2001/20/EC. It aims to establish a harmonised and efficient system for the submission, assessment, supervision, and reporting of clinical trials within the European Union, increasing transparency and availability of information on clinical trials and their results.
Under this regulation, all clinical trials must be registered, and their details made publicly available through the Clinical Trials Information System (CTIS). For documents to be published, CTIS offers the possibility to upload a version ‘for publication’ to provide redacted documents and a version ‘not for publication’ that may contain personal data and CCI, as needed for the scientific and regulatory review carried out by the Member States.
Sponsors must ensure that personal data are anonymised in order to safeguard the privacy of participants and personnel involved in clinical trials as well as Commercially Confidential Information (CCIs) are redacted in order to protect their legitimate economic interests.
FDA Clinical Trials Registration and Results Information Submission
Final Rule 42 CFR Part 11, effective on 18 January 2017, mandates the FDA requires the registration of “applicable clinical trials” (ACT) on the ClinicalTrials.gov portal and the submission of results information including the disclosure of Study Protocols and Statistical Analysis Plans.
ACTs generally include interventional studies (with one or more arms) of FDA-regulated drug, biological, or device products that meet one of the following conditions:
- The trial has one or more sites in the United States
- The trial is conducted under an FDA investigational new drug application or investigational device exemption
- The trial involves a drug, biological, or device product that is manufactured in the United States or its territories and is exported for research
The responsible party may redact personally identifiable information, or confidential commercial information (CCI) contained in the protocol or statistical analysis plan prior to submission, unless such information is otherwise required to be submitted under this part. A review should be conducted before submission in order to ensure no personal data or CCI are released to the public domain.
EMA Policy on access to documents (related to medicinal products for human and veterinary use) - POLICY/0043
The EMA Policy 0043 governs the rules the Agency applies to grant access to any document it holds on human and veterinary medicines coming from a request submitted by EU Citizens and natural or legal persons residing or having their registered office in an EU Member State.
In case of receiving an application for access to a document which originates from a third party (e.g., a MA holder or applicant), EMA will send a consultation to the author to discuss which content/information in these documents may need to be protected before the documents can be released. Third parties are invited to justify why some content/information is identified as commercially confidential and to indicate the personal data that need to be protected, within the timeframe of 5 working days.
European Public Assessment Reports (EPAR) and Risk Management Plan (RMP) publication
A European public assessment report (EPAR) is published for every human or veterinary medicine application that has been granted or refused a marketing authorisation. The EMA will liaise with the applicant to delete all commercially confidential information and personal data prior to publication.
From 20 October 2023, EMA is publishing RMPs (Risk Management Plans, main body and annexes 4 and 6) instead of summaries for all centrally authorized products in initial evaluations and RMP updates. The MAH (Marketing Authorisation Holder) is asked to provide a redacted version for publication considering personal data and commercial confidential information, although it is recommended that all necessary changes are already implemented via anonymisation/deletion in the RMP submitted for evaluation.
How can we help?
Profound understanding of regulatory framework
Specialised regulatory support and insights on complying with Transparency policies’ requirements – 1st-hand knowledge of the policies and their implementation
Liaison with Health authorities
EMA Pre-submission/HC Process Initiation Meeting preparation and attendance
Definition of redaction and anonimization strategy
Execution of anonymization and redaction processes, including Quality Check
Preparation of Anonymization reports
Assistance with justifications for Commercially Confidential Information
Publishing and submission activities (e.g. eCTD, CTIS, CT.gov)
We understand your challenges and have the expertise to guide you through transparency policies, offering tailored solutions with a deep understanding of the regulatory framework.
Schedule now a free meeting with our DATA DISCLOSURE experts!
Tell us about your project, challenges or doubts. We will be happy to assist you!
Related Content
Want to know more? Watch our webinar “Reactivation of EMA Policy 0070: Introduction and key recommendations for a successful Publication”
You can also have a look at this timeline that Asphalion experts have prepared for you to take into consideration the steps and timing regarding clinical data publication for new MAA or at the business case “Case Study Analysis: Transparency Policy 0070”.
Why Asphalion?
ASPHALION offers expert consulting, strategic advice, operational support and full outsourcing services for all drug development stages
ASPHALION’s experts have delivered solutions to over 1,000 Pharmaceutical, Biotechnological and Medical Technology companies from more than 50+ countries in over 5,000 projects covering non-clinical and clinical development, CMC, dossier writing, regulatory procedures, vigilance, eSubmissions and data management for both medicinal products and medical devices.
Quality

Memberships