AsphaSearch
Explore Globally, Read Less, Discover More!

AI-Powered Literature Search Tool for Smarter Pharmacovigilance
AsphaSearch is Asphalion’s AI-powered tool to perform global and local literature searches through a centralized, digital and regulatory-compliant platform.
- Extensively audited by top pharmaceutical companies
- Successfully inspected by regulatory authorities
- Delivers efficiency, traceability, and regulatory robustness to pharmacovigilance teams
AsphaSearch Key Benefits
Global & Local Search
Centralized access to both international sources and local regulatory databases.
Cost Reduction
Achieve up to 90% savings in Pharmacovigilance literature search activities.
Automated Efficiency
Minimize manual tasks and accelerate literature search with validated AI technology.
Full Traceability
Comprehensive digital logs to support audits and regulatory inspections.
Regulatory Compliance
Fully validated platform aligned with global pharmacovigilance guidelines.
Faster Insights
Detect safety signals earlier and make informed decisions more quickly.
Pharmacovigilance 2.0: Beyond automation
Artificial intelligence is reshaping Pharmacovigilance by increasing efficiency, improving collaboration, and enabling earlier risk detection and mitigation. Here’s how AsphaSearch contributes to a smarter, faster and more transparent Pharmacovigilance system:
Automatic processing of large volumes of adverse event report data
- Reduction of manual workload
- Improvement in response speed
- Increased efficiency in identifying adverse events
Identify patterns and correlations in data
- Early detection
Forecasting potential risks
- Improvements in anticipation and mitigation of risks
Improvements in communication & collaboration
- Faster & coordinated communication flow: regulators, manufacturers, and healthcare professionals
Transparency and availability of data
- All relevant information available for all parties involved in safety procedures
Smarter workflows start here!
Compare traditional processes with AsphaSearch AI-enhanced system:
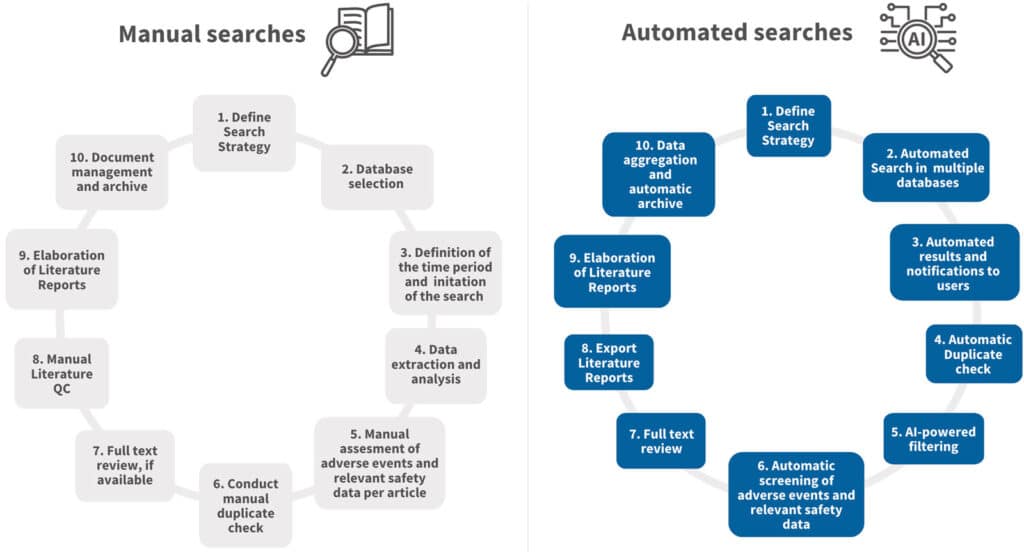
Our experts opinion
The use of software for conducting literature searches in PV can be considered a highly useful and beneficial tool for Pharmacovigilance Departments, improving the efficiency, speed, quality of work and profitability of the activity.
The incorporation of advanced AI tools presents a significant opportunity to enhance this crucial procedure in PV, which typically consumes substantial time and resources.
However, it is essential to guarantee the validity and transparency of AI algorithms and maintain an adequate balance between automation and expert supervision in the area of PV.
At Asphalion, we blend digital innovation with the deep expertise of our technical team, ensuring an optimal balance between technology and expert oversight. This synergy allows us to provide a service of complete trust to our clients, positioning us as a strategic pharmacovigilance partner
Let us show you how AsphaSearch can transform your literature search strategy
Why Asphalion?
ASPHALION offers expert consulting, strategic advice, operational support and full outsourcing services for all drug development stages
ASPHALION’s experts have delivered solutions to over 1,000 Pharmaceutical, Biotechnological and Medical Technology companies from more than 50+ countries in over 5,000 projects covering non-clinical and clinical development, CMC, dossier writing, regulatory procedures, vigilance, eSubmissions and data management for both medicinal products and medical devices.
Quality

Memberships



