Asphalion offers a full range of services to provide a comprehensive support for data compliance for:
Being a reference point for xEVMPD services, we are fully ready to support companies with:
Asphalion offers a full range of services to provide a comprehensive support for data compliance for:
Being a reference point for xEVMPD services, we are fully ready to support companies with:
Regulatory Operations: Data Management Solutions
At Asphalion we can give you support and help you navigate through the awareness process and work with you to be ISO IDMP compliant whether you have, need or can’t yet access a RIM system.
Importance of 3rd Acknowledgement management in the context of data migration to PMS
⚠️ Managing EMA’s 3rd ACK is a crucial step in the context of migrating xEVMPD data into PMS, aimed at preventing and minimizing inconsistent data input into both the PMS database and eAF.
If you need further information or wish to explore how we can assist you during this transition:
If you need further information or wish to explore how we can assist you during this transition:
If you need further information or wish to explore how we can assist you during this transition:
Uniquely identification of medical products
Schedule a free meeting!
Discuss your case with our experts and receive a valuable feedback
Downloads
Share
Why Asphalion?
xEVMPD Compliance
RIMS Implementation Expertise
Leading ISO IDMP Transition

Related Resources
DADI Webinar: Are you ready for Go-Live?
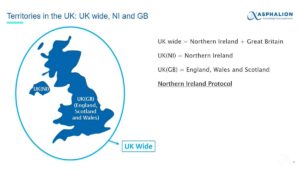
Regulatory Landscape in the UK: an update – Asphalion Webinar

RIM Implementation in times of IDMP – Asphalion Webinar

How to set-up your Post-Market Surveillance and Vigilance system for IVDs according to IVDR – Asphalion Webinar
Efficient IDMP implementation in different sized pharma companies: practical experience and FAQs – Asphalion Webinar
EUDAMED Module 2 Webinar with Lídia Cánovas and Francisco Rogríguez (Asphalion)

Asphalion provides dedicated services for all EUDAMED related activities with an
impact on manufacturers of medical devices

Beginning 15th September 2021
, FDA will enforce the
TechnicalRejection Criteria (TRC)
for Study Data by
CDER
and
CBER
.
A whitepaper about how to select your perfect RIMS

This white paper examines the business drivers behind this shift and three factors that are critical to maximizing thebenefits of a unified RIM solution.

The EU Regulatory Environment of Medical Device Software Development

EMA is holding a new public event with the purpose to provide a comprehensive understanding of Product Management Service (PMS) and its implications on other EMA digital services with the objective to introduce the core concepts of PMS, ensuring that all attendees have the chance to understand its implementation.

EMA held the first system demo of 2024, the ninth ever held by them as part of its Agile Network Portfolio management.

Harness the power of convenience with Asphalion’s scheduling tool – a streamlined way to arrange a meeting with our Regulatory and Safety experts.
Services
© Asphalion
Schedule here a free 30-minutes meeting with one of our consultants and tell us about your project, challenges or doubts.
We will be happy to assist you!

| Cookie | Duration | Description |
|---|---|---|
| cookielawinfo-checkbox-advertisement | 1 year | Set by the GDPR Cookie Consent plugin, this cookie records the user consent for the cookies in the "Advertisement" category. |
| cookielawinfo-checkbox-analytics | 1 year | Set by the GDPR Cookie Consent plugin, this cookie records the user consent for the cookies in the "Analytics" category. |
| cookielawinfo-checkbox-necessary | 1 year | Set by the GDPR Cookie Consent plugin, this cookie records the user consent for the cookies in the "Necessary" category. |
| cookielawinfo-checkbox-others | 1 year | Set by the GDPR Cookie Consent plugin, this cookie stores user consent for cookies in the category "Others". |
| CookieLawInfoConsent | 1 year | CookieYes sets this cookie to record the default button state of the corresponding category and the status of CCPA. It works only in coordination with the primary cookie. |
| elementor | never | The website's WordPress theme uses this cookie. It allows the website owner to implement or change the website's content in real-time. |
| viewed_cookie_policy | 1 year | The GDPR Cookie Consent plugin sets the cookie to store whether or not the user has consented to use cookies. It does not store any personal data. |
| wpEmojiSettingsSupports | session | WordPress sets this cookie when a user interacts with emojis on a WordPress site. It helps determine if the user's browser can display emojis properly. |
| Cookie | Duration | Description |
|---|---|---|
| _ga | 1 year 1 month 4 days | Google Analytics sets this cookie to calculate visitor, session and campaign data and track site usage for the site's analytics report. The cookie stores information anonymously and assigns a randomly generated number to recognise unique visitors. |
| _ga_* | 1 year 1 month 4 days | Google Analytics sets this cookie to store and count page views. |
| _gat_gtag_UA_* | 1 minute | Google Analytics sets this cookie to store a unique user ID. |
| _gid | 1 day | Google Analytics sets this cookie to store information on how visitors use a website while also creating an analytics report of the website's performance. Some of the collected data includes the number of visitors, their source, and the pages they visit anonymously. |
| Cookie | Duration | Description |
|---|---|---|
| VISITOR_INFO1_LIVE | 6 months | YouTube sets this cookie to measure bandwidth, determining whether the user gets the new or old player interface. |
| VISITOR_PRIVACY_METADATA | 6 months | YouTube sets this cookie to store the user's cookie consent state for the current domain. |
| YSC | session | Youtube sets this cookie to track the views of embedded videos on Youtube pages. |
| yt-remote-connected-devices | never | YouTube sets this cookie to store the user's video preferences using embedded YouTube videos. |
| yt-remote-device-id | never | YouTube sets this cookie to store the user's video preferences using embedded YouTube videos. |
| yt.innertube::nextId | never | YouTube sets this cookie to register a unique ID to store data on what videos from YouTube the user has seen. |
| yt.innertube::requests | never | YouTube sets this cookie to register a unique ID to store data on what videos from YouTube the user has seen. |