News and Events
Stay in touch with the latest news and upcoming events featuring Asphalion

WEBINAR | Clinical Trials transition to CTR/CTIS
Ready to equip yourself with first-hand information from our experts? Join us in our new free webinar, “Clinical Trials transition to CTR/CTIS”. All ongoing clinical trials in the EU must be transitioned to CTIS if they are expected to continue beyond 30 January 2025. Sponsors must consider the time required for Member States to complete the authorisation procedure, which can take up to three months. To help streamline the process, Member States will implement, where possible and up to 16 October 2024, an expedited procedure for transitioning trials to the CTR. For submission beyond that date, a prompt approval cannot
WEBINAR | Clinical Trials transition to CTR/CTIS
Ready to equip yourself with first-hand information from our experts? Join us in our new free webinar, “Clinical Trials transition to CTR/CTIS”. All ongoing clinical trials in the EU must be transitioned to CTIS if they are expected to continue beyond 30 January 2025. Sponsors must consider the time required for Member States to complete […]
More News & Events
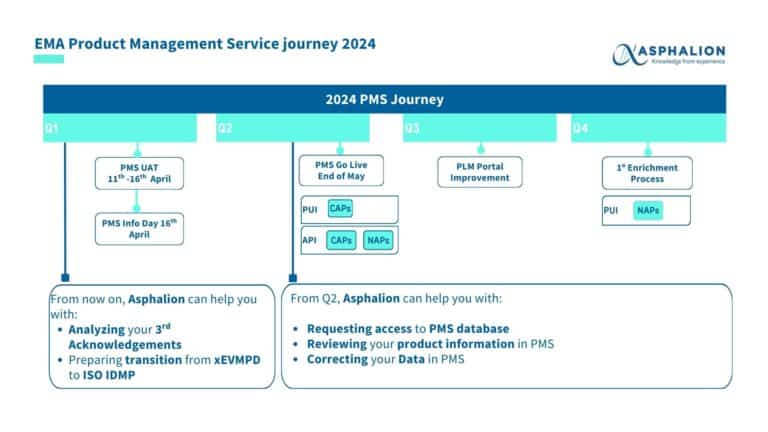
EVENT | EMA PMS Info Day
EMA is holding a new public event with the purpose to provide a comprehensive understanding of Product Management Service (PMS) and its implications on other EMA digital services with the objective to introduce the core concepts of PMS, ensuring that all attendees have the chance to understand its implementation.

EVENT | 20th Biosimilar Medicines Conference by MFE
During these two interactive days, actors of the healthcare community alongside world experts in biosimilar medicines will gather and debate the evolving biosimilar medicines landscape and emerging trends. Discussion will focus on sharing good practices and setting collective aims to achieve the depth, breadth and speed of the use of biosimilar medicines as a lever to untap their transformative value.

NEWS | New Insight on the Implementation of MDR Article 17
Article 17 of Regulation (EU) 2017/745 (Medical Device Regulation – MDR) regulates the reprocessing of single-use devices (SUDs) with relevance for the European Economic Area (EEA) which may only take place where permitted by national law and in accordance with this article.

New opening! | Pamplona office
At Asphalion, we are thrilled to announce the opening of our new office in Pamplona! This strategic

RESOURCES | Asphalion’s YouTube channel
Want to dive deeper into our services, expertise, and regulatory affairs? Head over to our YouTube channel

BUSINESS CASE | Transparency policy 0070
Have a look at this Business Case where Asphalion provided a leading global pharmaceutical company with full
Search or filter











